Green energy
Electrodeposition is a popular technique to produce metals, alloys and composite materials. The electrodeposition cell contains an electrolyte and two electrodes, namely the anode and cathode. The electrolyte as an ionic conductor is in the liquid state where the metal of interest (e.g. copper, silver, gold, zinc, nickel, chromium, etc.) participates in faradaic reduction reaction. The metal ion converts to metal atom at the cathode surface where a continuously growing metal deposit layer forms.
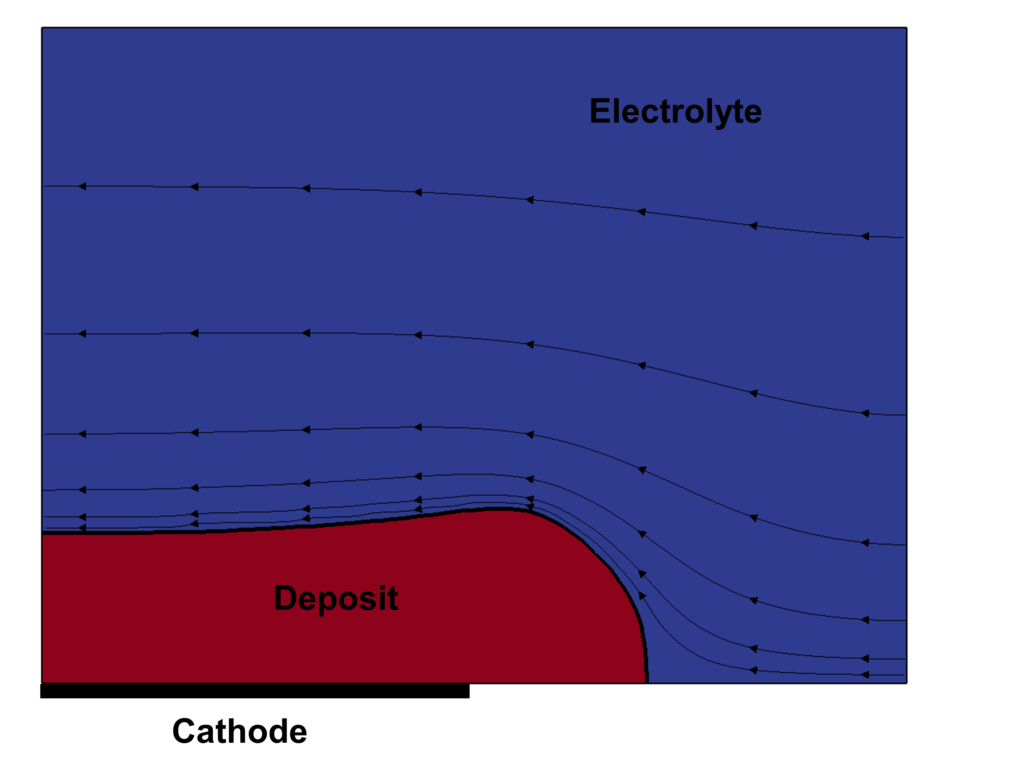
The evolution of the field structures in the region near cathode are shown:
including: volume fraction of deposit (β), contour of normalized electric potential and normalized equipotential surfaces (φ*), magnitude and streamlines of normalized electric current density (j*), the magnitude and streamlines of normalized velocity (u*).
We propose a novel approach using the volume of fluid method (VOF) to simulate transient shape change of the deposit front during electrodeposition. The deposit front moves forward as time proceeds. The animation shows the volume fraction of deposited material.
Electrodeposition is a popular technique to produce metals, alloys and composite materials. The electrodeposition cell contains an electrolyte and two electrodes, namely the anode and cathode. The electrolyte as an ionic conductor is in the liquid state where the metal of interest (e.g. copper, silver, gold, zinc, nickel, chromium, etc.) participates in faradaic reduction reaction. The metal ion converts to metal atom at the cathode surface where a continuously growing metal deposit layer forms.
Publications
1) Karimi-Sibaki E., Kharicha A., Vakhrushev A., Wu M., Ludwig A., Bohacek J.: Electrochem. Comm. 112 (2020) 106675. DOI: 10.1016/j.elecom.2020.106675
” A volume of fluid (VOF) method to model shape change during electrodeposition”
2) Karimi-Sibaki E., Kharicha A., Abdi M., Wu M., Ludiwg A., Bohacek J.: J. Electroch. Soc., 166 (2019) D521-D529. DOI: 10.1149/2.1241912jes
“A Dynamic Mesh Method to Model Shape Change during Electrodeposition.”

